Version History
 QMSCAPA Online User Guidance, 2.16.1 < What do the version numbers mean?
QMSCAPA™ is quality management software designed by a user group of quality professionals, including assessors, auditors, consultants and quality managers for quality management systems. If you have an idea or new feature that you would like to see in QMSCAPA please join our user group by completing our web form.
Do you have an idea for a new feature in QMSCAPA? Let is know by sending an email with your feature request to our support group. (sherlock @ abci-software.com). Click here to see what is planned on our list of new features.
12/23/2018 v1.57.10❑Enhanced the Job Title and Job Description reports. ❑Enhanced the Process Interaction and Sub-Process module to include: ❑Process Input and Output statements; ❑Key Document Identifiers (KDI); ❑Key Performance Indicators (KPI); ❑Reports for the Process Interaction with Standards. ❑Enhanced the Risk Mitigation Treatment Plan module to link to the Document Control Index (DCI). ❑Enhanced the Supplier Quality Report Card management module by adding a text field for messages to suppliers. ❑Enhanced the Quote and Contract Review log by adding a user defined Quote/Contract name field. ❑The Dashboard Table records are locked to prevent unintended deletions.
10/8/2018 v1.56.14❑Added access to CAPA attachments from the AIM/NCR Table. Therefore, electronic file attachments added in the Non-conformance Report shall be available directly from the CAPA form. ❑Enhanced the AIM/NCR Table to include a Reference # sort & search feature. ❑Enhanced the Monitoring and Measuring Device module. ❑Updated the Dashboard.
09/09/2018 v1.55.10❑Added two new Calibration Monitoring & Measuring Device reports. ❑Enhanced the Calibration Monitoring & Measuring Device and Instruments module. ❑Enhanced Supplier/Vendor Survey Questionnaires. ❑Updated the File Manager. ❑Updated the Print Preview module. 08/01/2018 v1.54.16❑Added a table for Training Program attachments. ❑Added a report journal for the review of contracts and quotes. ❑Enhanced the Customer Satisfaction Survey module. ❑Enhanced the Document Control Viewer application. ❑Enhanced the electronic document approval process. ❑Enhanced the Monitoring and Measuring Device Table to show the description field. ❑Enhanced the Risk Assessment form to allow the attachment of supporting documents. ❑Enhanced the risk boundaries lookup table of Risk Assessment templates. ❑Enhanced to ISO Control and Objective form to the expand Implementation Comments field. ❑Fixed the Supplier Evaluation tool to allow less than ten criterion.
07/01/2018 v1.53.14❑Enhanced the Personnel Training Reports. ❑Enhanced the Quote & Contract Review module to provide 'copy feature.' ❑Enhanced the Interested Party module to provide a direct link to the Risk and Opportunity assessments. ❑Enhanced the Risk Assessment & Management module to allow Interested Parties plus the expectations, objectives and requirements to be copied directly into the Risk Assessment module. ❑Enhanced the Performance Dashboard method for collection of data from monitoring objectives and process KPIs. ❑Enhanced the On-time Delivery module for recording batch deliveries/shipments. ❑Added to the Icon Menu bar an option to open the EasyStart Menu. ❑Added access to the Personnel Training Table to the EasyStart Menu.
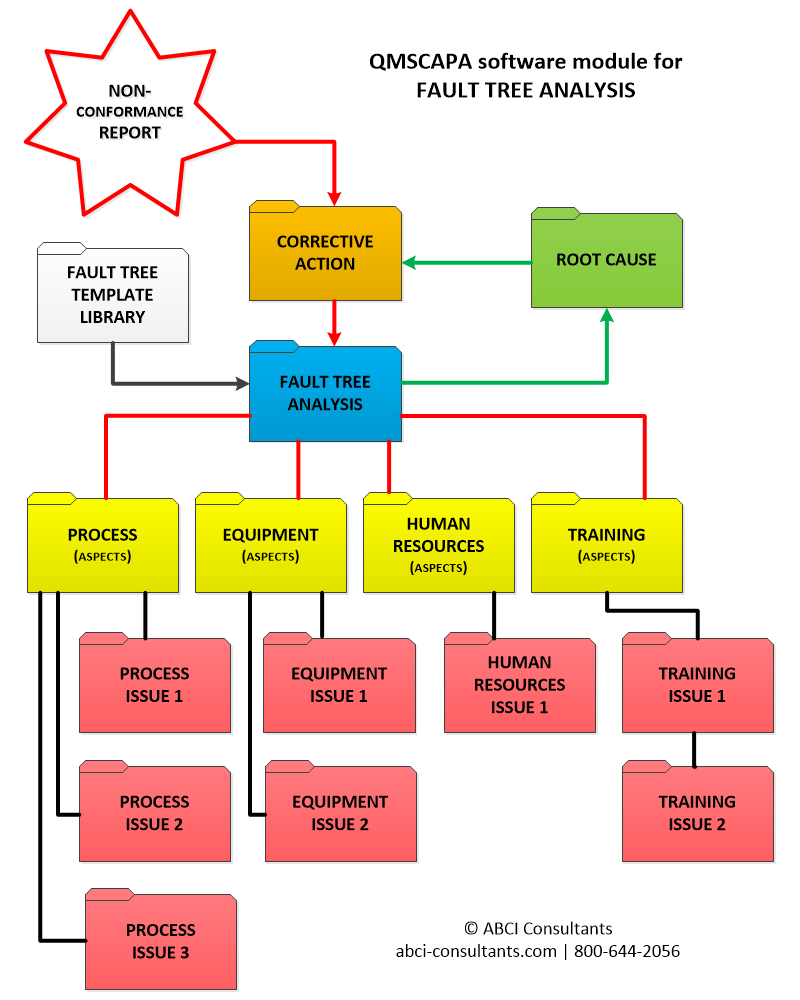
05/27/2018 v1.52.5❑Enhanced the Document Control Module and the automatic increment of the numeric version numbers. ❑Added a the Company default setting for 'Document Status' when documents are check as approved in the Document Control Module. ❑Enhanced to the Checklist Results form for selecting monitoring and measurement records. ❑Enhanced to the Quote and Contract review module. ❑Enhanced the Records Control Index module. ❑Enhanced the Supplier/Vendor Survey Questionnaire Template management module. ❑Fixed the Fault-tree Template copy feature, which is used to create a Fault-tree Analysis used in determining the 'root cause' for more effective Corrective Actions.
04/20/2018 v1.51.11❑Added RMA Journal report. ❑Added a Copy to Excel feature to the Monitoring and Measurement Device journal for calibrations and maintenance. ❑Enhanced the QMSCAPA user setup for e-signatures. ❑Added a new feature to provide a journal and reports for Customer Quote/Contract/Purchase Order review. ❑The Risk Assessment form was updated to require a Risk Assessment Set Identifier (RA Set Id). ❑Updated Process KPI Line/Bar Charts a) and b) to include the KPI Label Set Id and Name. ❑Updated the Training Schedule module.
❑Enhanced the default form selection method for printing CAPAs and AIM/NCRs, which allows these forms to print even if the user deletes the form listing in the Document Control Index. ❑Corrected the button text for the Easy Start menu. ❑Added an employee/member signature line to the Security Aspect Permissions report template. ❑Fixed the page numbering for the Objective & Control Methods report. ❑The Checklist Results database now supports the multiple types of measurements. ❑A Fault Tree Analysis module is added for determining root cause analysis and linked to a Corrective Action record. ❑Updated the Document Control Index Viewer (DCIviewer.exe)
11/28/2017 v1.48.5❑The Monitoring and Measurement Device table may be copied to Excel. ❑Electronic approval of documents may only be executed by the logged-in authorized signer. ❑Updated the Windows 10 manifest. ❑Updated the Digital Code Signing Certificates for the QMSCAPA.exe and installer.exe
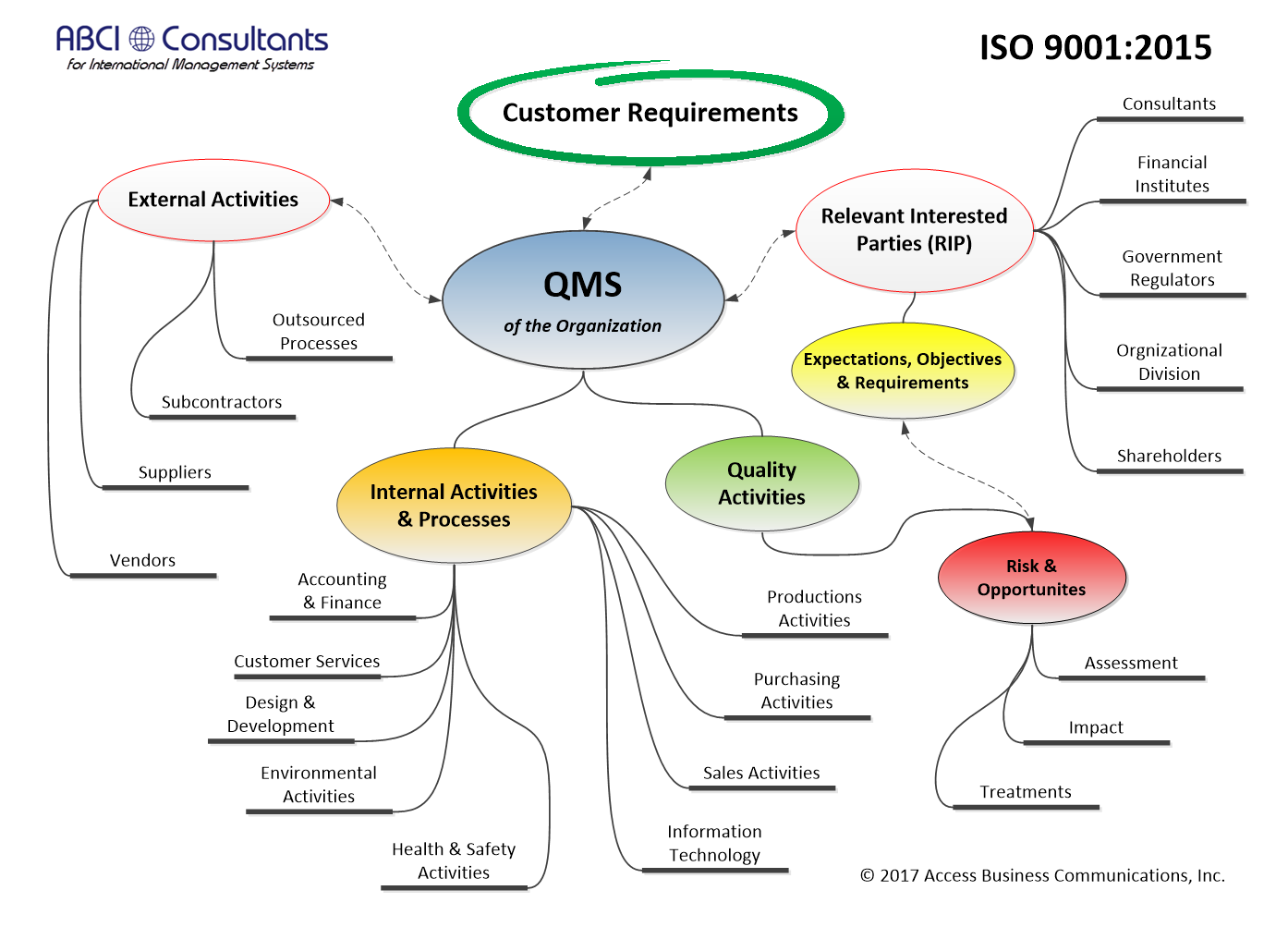
09/01/2017 v1.47.26❑Added the NIST 800-171 Basic Security and Derived Security requirements to the Controls Table. ❑Enhanced the Objectives and Controls Table to include a comments field for controls not yet implemented. ❑Enhanced the Human Resources Table. ❑Enhanced the Risk Management module to allow a direct relationship from the risk element to the control. ❑Added an Objectives and Controls report for Controls not implemented.
07/02/2017 v1.46.12❑Added a control to copy the Customer Satisfaction Survey records to Excel. ❑Added a link from the Risk Assessment module to RIP's Expectations and/or Requirements table. ❑Enhanced the Document Control module by adding approval requirements and a method for verifying document approval. a.Enhanced the Document Approval and Revision History module. b.Added a module to allow access to only unapproved documents that may be approved electronically by authorized signers.
 05/12/2017 v1.43.5 QMSCAPA EasyStart Menu option ❑Added a Relevant Interested Parties (RIP) table, which includes: ❑Library lookup table for stored RIP Expectations, Objectives and/or Requirements ❑1 to 999 Expectations, Objectives and/or Requirements may be linked to a RIP record ❑Each Expectation, Objective and/or Requirement may be directly linked a Risk Assessment and Impact Statement ❑RIP Reports may be printed with or without the related Expectations, Objectives and/or Requirements
03/12/2017 v1.42.2❑The Document Control Module was enhanced to include a 'Login User Code' field to the Document Index, Change History Log and Approval Journal Records. ❑Also, Records Control Module was enhanced to include a 'Login User Code' field to the Record Index and Record Approval Journal. ❑The Document Control Browse Table now displays the Documents, Change History and the Approvals in the same window. ❑The Human Resources Module (Employee Table) has been enhanced with a User Profile Options tab to set folder preferences for saving PDF attachments. Also, options to open the Easy Start Menu and to open the Action Task Reminders module at Login have been created per user. ❑The Product Configuration Module has been enhanced with additional Configuration Management reports and the QC Checklist may be selected and linked from the the Product Configuration Edit Form. ❑The Email Assistant used in the Customer Feed-back, CAPA and AIM NC Reports modules has been enhanced to allow users with web-based email clients to paste the email construct text directly into the client. ❑The Objectives and Controls Table has been enhanced when used with a Risk Assessment Module. ❑The Print Preview Module was enhanced to improve the Printer Selection options and to Save as PDF. ❑The Quality KPI Module was enhanced to improve ease of use.
12/23/2016 v1.40.11❑Added an option to copy the ISO Management Standard clauses to create derivatives from similar Standards, e.g. ISO 9001:2015 to AS9100D. ❑Enhanced the Report Preview features and options. ❑Updated the Supplier Survey report template. ❑Added a separate application (DCIViewer) for providing access to read-only documented information at the point of use. 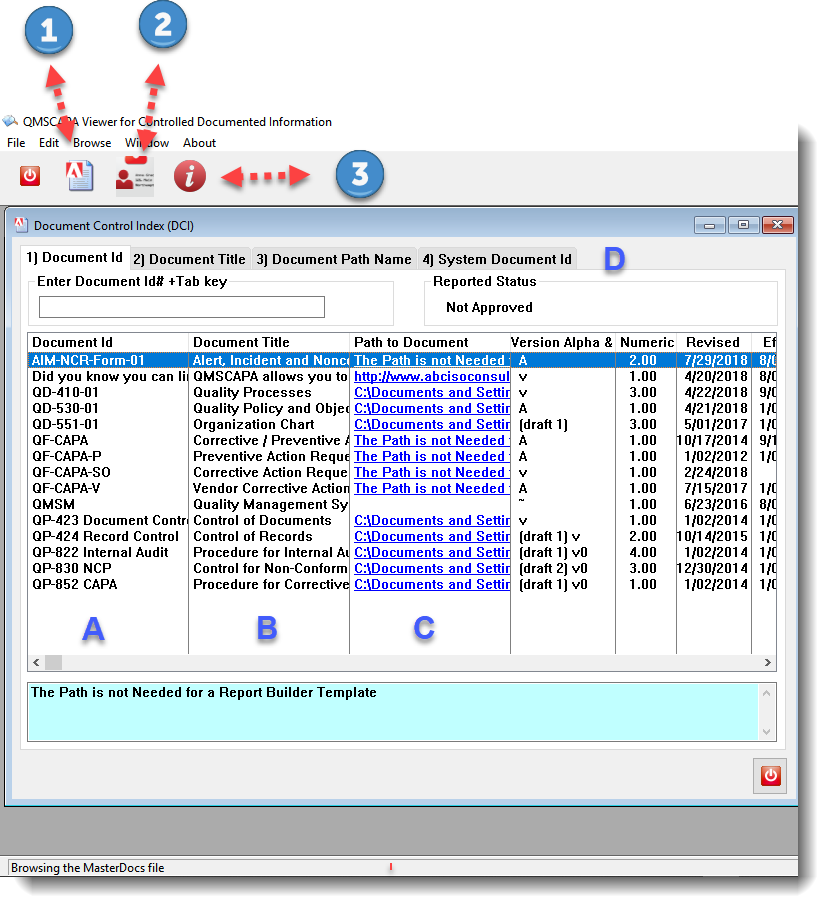
07/05/2016 v1.39.17 ❑Added an Easy Start Menu option for new users that provides an alphabetical listing of frequently used modules. ❑Added an image control to each Checklist Item record, which allows images to be included with each checklist item. ❑Added a Product Configuration Management method and reports. ❑Added new Product and Services reports. ❑Added new Chemical and Material database and reporting module, which provides support for the OSHA Hazard Communication Standard for Safety Data Sheets (SDS) revised in 2012, which includes 16 standardized sections. ❑Introduces a new method for Product Life Cycle Planning and Evaluations, which supports product life-cycle based thinking in ISO 14001:2015. ❑Added Spell-check for the Windows Edit Form for Safety Data Sheets. ❑Added a Label Set Name Sort Tab to the KPI Label Set Table.
05/15/2016 v1.38.8 ❑Added an advanced CAPA query and search feature to assist with CAPA analysis. ❑Added an assignment tool to assist with adding a personnel group to a Training Schedule. ❑Added a table to link Training Program requirements to Job Titles. ❑Updated the Graphical Charting driver.
04/24/2016 v1.37.6 ❑Enhanced the Performance Dashboard and Report. ❑Added a Microsoft Outlook integration link from the QMSCAPA TASK table to Microsoft's Outlook TASK table. ❑Add the ISO 9001:2015 clauses to aid internal audit planning and writing audit findings.
02/01/2016 v1.36.14 ❑Added new virtual printer drivers for generating reports as ❑Microsoft Excel file (requires Microsoft Excel) ❑Microsoft Windows Metafile (WMF) (requires Microsoft Word) ❑Portable Document File (PDF) ❑Rich Text File (RTF) ❑Added ISO Objectives and Controls table ❑Added ISO 27001 Statement of Applicability report
02/01/2016 v1.35.8 ❑Enhanced the Supplier /Vendor Evaluation reports ❑Enhanced the Customer forms and table ❑Added a Quality Targets Table, which may be used to set On-time Delivery and Customer Satisfaction targets per customer record. ❑Added a Customer Reports group to report the results achieved from activities used to measure on-time delivery (OTD) and customer satisfaction (CS). ❑Added to the CAPA form and database a separate Containment Actions field.
01/15/2016 v1.34.15 ❑Enhanced the Performance Dashboard. ❑Enhanced the Product/Service Database module. ❑Enhanced the Process KPI reports. ❑Enhanced the User-Defined Help (UDH) module. ❑Enhanced the Supplier Survey and Evaluation module
12/23/2015 v1.34.8 ❑Enhanced the Security Access and Security Aspects Reports. ❑Added an optional Record Lock to the Document Control Index to prevent unintended deletion of records. ❑Enhanced the Internal Audit Schedule Module and Reports to include Process information.
12/17/2015 v1.34.6 ❑Added an optional Record Lock to the Document Control Index to prevent unintended deletion of records. ❑Enhanced the Internal Audit Schedule Module and Reports to include Process information ❑Added Document Approval Journal to Document Revision History ❑Added Document Approval Journal Reports ❑Enhanced the Document Approval Process to comply with FDA Title 21, Sub-Chapter A, Part 11. ❑Corrected E-Signature sort order on HR Table.
12/04/2015 v1.33.9 ❑Alert Messages and NC Reporting Module ❑Added three new AIM/NC Reports ❑Added to the AIM/NC form a user-defined look-up table for disposition ❑Enhanced the AIM NC Module Reports ❑Enhanced the CAPA Log Reports ❑Enhanced the KPI reports ❑Enhanced the Preventive Action Reports
11/05/2015 v1.33.4 ❑Enhanced the AIM NC Module Reports ❑Enhanced the Records Control Index Module ❑Records Control Form has new fields to ... ❑allow the record to be locked; ❑record the date the record was locked, and; ❑identify the user that performed the lock, and; ❑identify the position required for a record approval. ❑Records Table Structure now includes .. ❑Records Group Name, and; ❑Chronological listing of Records in the Group, and; ❑Records Approval Module that includes the Date & Time a Record is approved, and; ❑an option to lock the record to prevent unauthorized changes to approval records, ❑which conforms to FDA Title 21, Sub-Chapter A, Part 11 ❑Added support for electronic signatures. ❑Enhanced the Records Index Reports, including a ❑Records Approval Journal.
10/09/2015 v1.32.16 ❑Enhanced the AIM NC Reporting Module, which includes new report templates ❑Enhanced the Quality Dashboard ❑Enhanced the CAPA Task List report template ❑Added a new Export to Excel feature for key files ❑Added a new Import from Excel Wizard ❑Added batch entry feature for the On-time Delivery data from external programs ❑Added a copy to Excel feature to the Document Control Index ❑Added a copy to Excel feature to the Records Control Index ❑Enhanced the Customer Table ❑Activated a Customer Type field and validation table for classification of customers. ❑Enhanced the Risk Assessment and Management Module ❑Added Risk Impact Statements to the Risk Aspect module ❑Added Risk Response and Risk Status fields to the Risk Aspects module ❑Added Risk Type field to the Risk Assessment module ❑Added a Applicable Control Table to the Risk Assessment module ❑Enhanced the default Monitoring & Measurement Device reports.
06/01/2015 v1.31.5 ❑Adjusted CWG group filter for CAPA display ❑Restored copy feature in the On-time Delivery Calculator ❑Enhanced the Supply Chain Management module ❑Added a sample Supplier /Vendor Survey Questionnaire ❑Click here to download a PDF copy of the QMSCAPA Supplier Survey Questionnaire
04/10/2015 v1.30.03 ❑Enhanced the Document Version History log to sync with the document Current Revision date. ❑Enhanced the Monitoring & Measuring Device/Equipment Table to include intervals for scheduling calibrations and maintenance ❑Added two new reports for scheduling Monitoring & Measuring Devices for calibration or maintenance ❑Task may be linked to the AIM-NCR form ❑Added additional AIM-NCR reports ❑Added a Training Sign-in & Sign-out sheet ❑Added the Job Title to the employee training look-up table ❑Added spell checking to the Customer Satisfaction Survey Sets ❑Report templates have been updated a)The Reports Directory now follows the user select Data Directory. |
Online Internal Auditor Training Course ISO Management Systems  +1 800 644 2056
|